Supercharged with inventiveness and expertise: fumed metal oxides from Evonik
70 years after the start of its success story and proven applications in powder coatings, illuminants, electronics and photocatalysis, Evonik's AEROXIDE® fumed metal oxides have now taken on a key role in Evonik's Next Generation Solutions for electromobility: Aluminum oxides and titanium dioxides from the product family accelerate the global progress of climate-friendly e-mobility by increasing the safety, performance, and longevity of lithium-ion batteries for electric vehicles.
As early as the 1950s, the automotive industry was an impetus for experiments in the production of alumina at Degussa, a predecessor company of Evonik. But the findings and advances in the production of fumed metal oxides in the so-called flame hydrolysis process initially led to completely different, surprising applications. The process had been developed and patented by Degussa in 1941 for the production of silicon dioxide. Driven by a thirst for research, the chemists then experimented with other substances as well. The same principle could then be used to produce fumed metal oxides.
High-temperature flame hydrolysis

Gaseous metal chlorides are fed into the oxyhydrogen flame. Fumed oxides are generated by subsequent hydrolysis reaction taking place at high temperature. AEROXIDE® products from Evonik are characterized by their high chemical purity and large specific surface area. The size and morphology of the particles can be specifically defined during the process.
A passion for metal oxides
The success story, initially with titanium dioxide, begins with a telex dated December 10, 1952: “The TiO2 trials started a few days ago and have basically confirmed the option of working with a mixture burner and flame tube,” stated chemist Dr Ernst Wagner in a telegraph to the Degussa management. In that same telegraph message, Dr Wagner also announced the imminent start of the flame tube test plant for alumina at the site in Rheinfelden, Germany. Due to delays in the delivery of apparatus parts for the alumina trials, the pyrolysis of titanium dioxide was brought forward - this would soon develop into a lucrative business for Degussa.
Dr Ernst Wagner (1907 – 1961)

In the 1950s, Dr Wagner was the chief chemist at Degussa's Rheinfelden site. He continued the pioneering work of Dr Harry Kloepfer, who in 1942 had produced “white carbon black” - the fumed silica AEROSIL® - for the first time. Dr. Wagner revised Kloepfer’s combustion chamber process and replaced it in 1952 with the flame tube process – the basic principle behind the process is still used today for the production of AEROSIL® fumed silica and AEROXIDE® products.
In February 1953, the hydrolysis of alumina in the flame tube process also succeeded, and Dr Wagner was pleased with "a light and fluffy snow-white product that looks deceptively similar to Aerosil fumed silica". Unlike AEROSIL® fumed silica, fumed alumina – besides other physico-chemical properties – has an electrostatically positive surface charge, which creates and allows many possible applications for the new product.
However, everything initially focused on novel fillers for car tires – as an alternative to carbon black. The results of the alumina pyrolysis were eagerly awaited in the company because the tire manufacturer Continental was very interested in the substance as a filler for rubber compounds. In the 1950s, there was great demand for light-colored, active fillers with pronounced reinforcing properties in the rubber industry. Tire manufacturers are placing increasing emphasis on high wear resistance, abrasion resistance, and favorable aging behavior.
Applications of AEROXIDE® fumed metal oxides

AEROXIDE® Alumina improve the processing properties, quality, appearance, and performance of powder coatings.

AEROXIDE® Alu C acts as an inorganic binder in the luminescent layer of fluorescent lamps, preventing mercury from penetrating the protective layer of the glass tubes. This improves luminescence and increases the life span of fluorescent lamps, while also reducing energy costs.

AEROXIDE® TiO2 PF2 improves the thermal stability of silicones.

Surface-treated AEROXIDE® fumed titanium dioxide has been used for a while as a mineral UV filter in sunscreen formulations, increasing photostability and water resistance.

AEROXIDE® fumed aluminum oxide in microporous paper coatings brings about high-gloss surfaces, short drying time for the ink, photo-realistic resolution, and high color brilliance.

Toner particles treated with AEROXIDE® TiO2 have improved flow properties, thanks to better control of the triboelectric effects in toner powders.

As a free-flow additive in detection powders, AEROXIDE® Alu C contributes to aircraft safety. Powders with magnetizable particles and a fluorescent substance are used to check turbine parts for microcracks.

AEROXIDE® TiO2 as an active component in photocatalytic reactions can make a sustainable contribution to air purification by binding air pollutants.
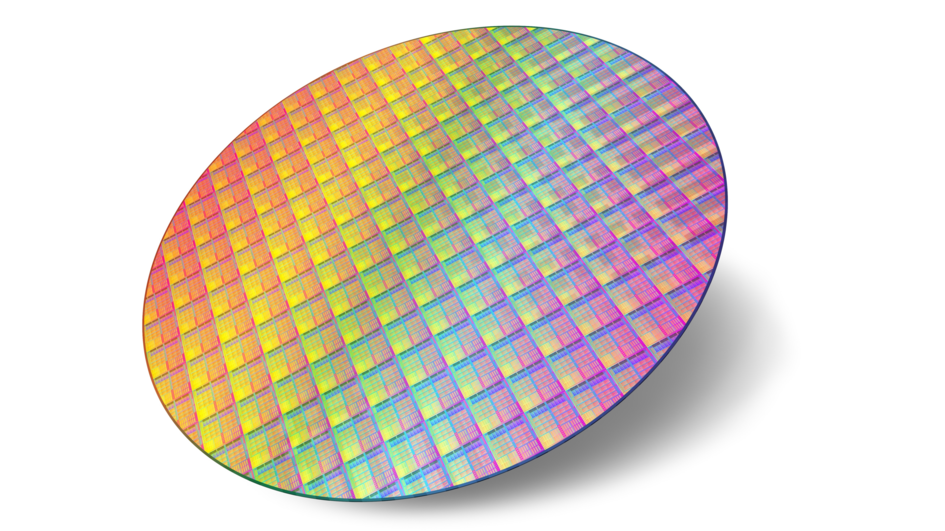
AEROXIDE® particles are used as abrasives for chemical-mechanical polishing (CMP) of wafers for microchips.

AEROXIDE® fumed metal oxides increase the safety, durability, and performance of lithium-ion batteries for electric vehicles and electronics.

AEROXIDE® TiO2 is a potential catalyst material in the production of synthetic fuels from renewable energies via Fischer-Tropsch synthesis.
Now things have to move fast
The researchers and developers at Degussa were under great time pressure because the competition was also working on processes to produce rubber-active alumina. Now, of all times, the supply of the starting material was running low at the Rheinfelden plant. Shortly after the successful test run, an urgent request was made to the purchasing department to order five metric tons of aluminum chloride as quickly as possible from BASF – incidentally, still one of the main suppliers of this raw material - as the stocks will only last for another four days.
However: aluminum oxide is no longer of interest as a potential tire filler, by now. In the rubber and tire industry, a silica product made by the company will experience a meteoric rise instead: ULTRASIL®.
From Alu C and TiO2 P 25 to the AEROXIDE® brand
After the breakthrough of 1953, nothing stood in the way of producing fumed alumina on an industrial scale, and so Dr. Wagner sent a proposed name “so that the product could be christened and launched on the market right away”: "ALUSOL," derived from alumina aerosol. But until the current AEROXIDE® trademark was registered on February 3, 2003, finding a name continued to cause headaches for patent attorneys and marketing experts. "ALUSOL" and "ALUTEX" were rejected because Degussa's patent department feared disputes with other chemical companies that had registered similar-sounding product names. For this reason, the names Alumina C and Titanium Dioxide P 25 were retained for a long time.
Metal oxides product family

Bags with the former German product names Aluminiumoxid C and Titandioxid P 25 from the 1990s. By the way: The “C” in Alu C stands the roman numeral for 100, which is the BET surface area of this alumina product in m2/g.

Since 2003, the metal oxides product family has carried the brand name AEROXIDE®.
First applications for metal oxides
In February 1959, the highly dispersible metal oxides were presented to Degussa's sales representatives. These metal oxides had proven themselves in the first industrial applications six years after the first test runs in the flame tube: A lighting manufacturer in the U.S. that used alumina P 110 C 1 to coat fluorescent tubes was able to save a production step and achieves higher initial brightness. Another U.S. company values it as an active filler for acrylonitrile rubber. And titanium dioxide P 110 was discovered by the German paint and coatings industry as a process auxiliary: Degussa was delighted to receive orders from the market leader, which was very satisfied with the quality.
In May 1966, news from the USA attracted attention: The Food and Drug Association (FDA) approved titanium dioxide as an additive for coloring food and pharmaceutical products. Initially, this was also of interest to Degussa. However, because the highly dispersed TiO2 products were not suitable for white coloring, the chemists modified the flame tube process to produce titanium dioxide with larger particles and pigment properties on a test basis. But this was not pursued further due to economic considerations. Instead, the properties of Degussa's fine-particle titanium dioxide were becoming increasingly important in new areas of application, for example as a process auxiliary and UV filter in cosmetics.
Sun protection for skin and materials
Some pigments can reflect, scatter, and absorb harmful UV radiation. Titanium dioxide has at times been used as a mineral UV filter in sunscreens in Europe roughly between 1995 and 2010 and earlier in Japan, when sun blockers with very high sunlight protection factors became increasingly popular. The TEGO® Sun product line includes surface-treated titanium dioxide for sunscreen formulations with increased photostability and water resistance. What protects skin from sun damage also protects materials against UV radiation and heat exposure, and TiO2 also develops this effect as an additive for surface coatings.
AEROXIDE® Alu C shows positive effect in environmentally friendly coatings
Meanwhile, aluminum oxide had assumed a unique position in powder coatings. In the 1960s, the electrostatic powder coating process was developed. Since then, fumed alumina has improved the processability and quality, appearance, and performance of powder coatings through their positive electrostatic charge. In particular, the flow properties have been decisively improved, which in results in a very homogeneous distribution in the powder coating. AEROXIDE® products from Evonik continue to make an important contribution to environmentally friendly, solvent-free coating systems in this industry.

The antistatic effect also made alumina an effective free flow additive for polymer powders and granules that become negatively charged by friction and adhere to the walls of containers. Alumina neutralizes the charge and facilitates handling and dosing, for example of PVC powder. In so-called detection powders, it even contributes to aircraft safety: Powders with magnetizable particles and a fluorescent substance were used to check turbine parts for microcracks. Damaged areas become visible under UV light.
Electronics boom causes demand for metal oxides to rise rapidly
With the electronics boom in offices and households starting in the mid-1980s, new applications for fumed metal oxides emerged, for example for printing technology. Alumina provides color brilliance, photorealistic resolution and a water-resistant surface in microporous coatings for ink-jet paper. And titanium dioxide improves the capabilities and stabilizes the electrostatic charge of toner powders for copiers and laser printers. Microchips are at the heart of all electronic devices, and the global semiconductor industry is growing rapidly. A crucial process step in wafer production is chemical mechanical polishing (CMP) – also using abrasive AEROXIDE® particles.
Alumina helps save energy
Since the 1990s, Europe has been phasing out the old incandescent bulbs because they consume an enormous amount of electricity. In the new, so-called energy-saving light bulbs, Alu C is indispensable: As in fluorescent tubes, it acts as an inorganic binder, allowing the fluorescent coating to adhere to the glass. In addition, it forms a protective layer that prevents mercury atoms from diffusing into the glass. In this way, the amount of mercury can be reduced to a minimum and the service life of the fluorescent lamps significantly extended.
Titanium dioxide purifies the air
Titanium dioxides produced by the flame hydrolysis process surprised researchers with their very high photoactivity. This is due to the unique structure and very high specific surface area, which is completely available for catalytic reactions. With this property, AEROXIDE® titanium dioxide can make a sustainable contribution to air purification by binding air pollutants via photocatalytic active wall paints, facade coatings, roof tiles, paving stones and other building materials: Toxic nitrogen oxide (NOx) on the surface of the titanium dioxide is converted to water-soluble nitrate, which is washed away by rain.

AEROXIDE® metal oxides drive the NextGen Mobility
And once again, AEROXIDE® metal oxides take on an important function in sustainable technologies for a greener future: they contribute to a climate-friendly mobility by increasing the performance safety and service life of electric vehicle (EV) lithium-ion batteries (LIBs).
Soon, the next generation of EV batteries will become even more efficient, thereby enabling greater driving distances. EV manufacturers are exploring new battery pack designs that eliminate the module housings, such as “cell-to-pack” (CTP) technology. This approach reduces weight, increases energy density, lowers cost, and simplifies manufacturing. However, it also imposes more demanding environmental and mechanical performance specifications on the battery pack. Innovative formulations for thermally stable silicones, thermal conductive adhesives, and thermal insulation materials are needed to address these challenges.
Alongside battery-electric drives, CO2-neutral e-fuels are discussed as an alternative technology for greener mobility. AEROXIDE® can also make a relevant contribution in the power-to-liquid-process: Titanium dioxide is a potential catalyst material in the production of synthetic fuels from renewable energies via Fischer-Tropsch synthesis. This technology could become significant in the future to produce sustainable kerosene for aircraft.
AEROXIDE® metal oxides are ready for these challenges, and those to come in future. Evonik provides inventive spirit, knowledge, experience, and high-performance products for this purpose – powering a greener future!




