
FAQ
Silica and STOT RE 1
For experts: More detailed, scientific answers are in italics
Questions regarding the CLH process
In the European Union, all substances or mixtures that are manufactured or imported in quantities of above one metric ton a year must be registered with the European Chemicals Agency (ECHA) according to Art. 6 REACH Regulation. Part of the procedure is a substance evaluation (CLH), which aims to clarify whether a substance constitutes a risk to human health or the environment.
A substance evaluation of synthetic amorphous silica (SAS), a type of silicon dioxide (SiO2), was initiated in 2013. The final report, published in 2021, left doubts as to the complete safety of the substance based on inhalation studies. This led to a review of synthetic amorphous silica in the CLH process. Subsequently, the competent authorities (CA) recommended a harmonized classification for synthetic amorphous silica as STOT RE 1 (H372, signal word “Danger”).
STOT means “specific target organ toxicity” and is a hazard class of chemicals for which Regulation (EC) No. 1272/2008 of December 16, 2008 (CLP Regulation) prescribes specific labeling and packaging.
STOT RE consists of two hazard categories: STOT RE 1 (H372, signal word “Danger”) and STOT RE 2 (H373, signal word “Warning”).

Substances with a classification of STOT RE 1 or STOT RE 2 (inhalation) must be labeled with the GHS hazard symbol 08 and the corresponding signal word “Danger” or “Warning.”
The reference H372 means: Causes damage to (respiratory tract/inhalation) through prolonged or repeated exposure if inhaled.
Harmonized classification and labeling may be proposed for substances not currently listed in Annex VI of CLP and for substances for which a harmonized classification exists but requires modification due to new information, new scientific or technical developments, changes in classification criteria or re-evaluation of existing data.
The respective competent authority (CA) of EU Member States, manufacturers, importers and downstream users can submit a proposal for the harmonized classification and labeling (CLH proposal) of a substance to the European Chemicals Agency (ECHA). This can be done in three different situations:
- if the substance is either carcinogenic, mutagenic, toxic for reproduction or an inhalation allergen,
- if there is a justification that the classification of a substance in other hazard classes is necessary at EU level,
- if one or more new hazard classes need to be added to an existing entry under the above conditions.

It is available for download as a large image in the area to the right!
GHS stands for Globally Harmonized System. CLP stands for the Regulation on Classification, Labeling and Packaging of substances and mixtures.
The CLP Regulation governs the classification, labeling and packaging of chemicals and implements the internationally valid Globally Harmonized System (GHS) of the United Nations in the EU. The GHS serves as an internationally uniform basis for classification, labeling and packaging.
The main aim of the CLP Regulation is to inform stakeholders in the supply chain about the potential harmful effects of substances and mixtures by classifying and labeling them accordingly.
In the European Union, most substances and formulations are subject to labeling requirements that list the ingredients and declare the hazard potential. Products with a substance classified as STOT RE 1 in a concentration of more than one percent by weight (>1 wt %) must be labeled with the GHS symbol 08 and the corresponding reference "Causes damage to organs (lungs) through prolonged or repeated exposure if inhaled."
For cosmetics and food, particularly strict regulations apply to the labeling of packaging, which serve to protect consumers.
Although the European Union is taking on a pioneering role and wants to implement the objectives of the European Green Deal internationally, many measures are currently not being adapted outside Europe. The responsible state ministries of several industrial countries have already raised doubts about the classification of individual substances. There are already numerous substances that need to be labeled differently in the EU than in countries outside the EU.
Questions regarding silica in general
Silica is the common name for silicon dioxide (SiO2). SiO2 is very common in nature, for example, as crystalline silica in quartz or grains of sand. Another form of silica, amorphous silica, is found in human and animal organisms as well as in plants. It gives horsetail grass its stability, for example.
By the way: The two components in this compound, oxygen and silicon (approx. 27 %), are among the most common elements in the Earth's crust when measured by weight.
The industry can produce silicon dioxide synthetically through different processes. The rather inhomogeneous natural raw material is converted into silica products with a constantly high purity and quality for a wide spectrum of specific applications in numerous industries. In simple terms, sand is turned into a valuable high-purity material. Only in this pure form is synthetic amorphous silica an essential additive for countless industrial applications.
Evonik produces only synthetic amorphous silica, or SAS.

The various SAS types produced by Evonik differ in terms of their physicochemical features, such as particle size, density or surface treatment. That's why Evonik offers tailor-made silica products with specific properties for many different applications.
Silica types with crystalline and amorphous structure are found in nature and are also produced synthetically.
Quartz as it is in rocks or as grains of sand e.g. is a crystalline silica, whereas the silica which is incorporated in plants is amorphous. The structure of this incorporated silica can look very similar to the structure of Synthetic Amorphous Silica (SAS). While crystalline silica has an orderly lattice structure, amorphous silica has a randomized structure. Consequently, it differs from crystalline SiO2 not only in physical terms but also as regards its toxicological properties. There are no hazards associated with amorphous silica.
SAS is the abbreviation for synthetic amorphous silica. "Synthetic" means that the silica was produced in an industrial process. "Amorphous" means that the atoms are not organized in a regular lattice pattern, but in irregular shapes.
In terms of its structure, synthetically produced silica is indistinguishable from natural amorphous silica.
Synthetic amorphous silica is recognized as a nature-identical, sustainable and safe material that plays an important role in many different applications. Evonik produces only synthetic amorphous silica. This distinction is very important to set it apart from crystalline silica.
The industry predominantly uses two different routes to produce SAS product groups with specific properties: Precipitated silica and silica gels are produced from an aqueous solution, while fumed silica is created in a hydrogen flame. Both product groups – precipitated silica and fumed silica – belong to the amorphous silica types. Evonik markets its fumed silica under the brand name AEROSIL® and precipitated silicas under the SIPERNAT®, SPHERILEX®, ULTRASIL®, ZEODENT® and ZEOFREE® brands.
When talking about silica or silicon dioxide in general, it is important to understand the difference between amorphous and crystalline silica. Both types occur in nature: Quartz rock, for example, is a primary form of crystalline silica whose structure resembles a regular lattice framework. Silica is also incorporated in plants such as horsetail and rice. his type is called amorphous silica because it has an irregular structure. Synthetic amorphous silica (SAS) created in an industrial process has an identical structure to that of amorphous silica in plants.
Consequently, it differs from crystalline silica not only in physical terms but also regarding its toxicological properties: Breathing in dust of crystalline silica, for example in mining, stonemasonry, and sandblasting, can cause the lung disease silicosis, which leads to irreversible damage of the lung tissue. On the other hand, it has been proven that amorphous silica and synthetic amorphous silica do not cause silicosis.
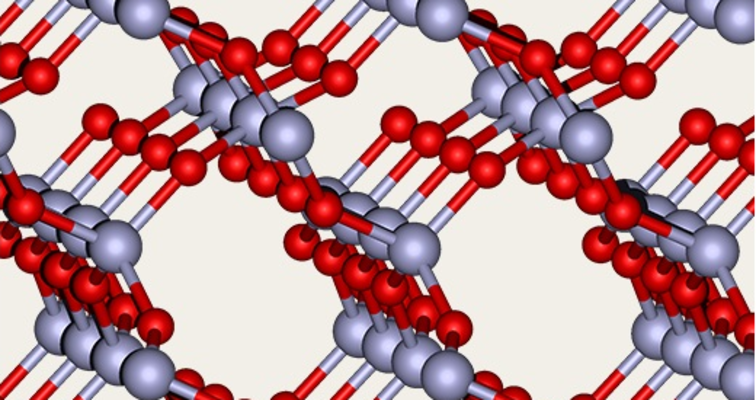
The atoms are arranged in a regular lattice structure
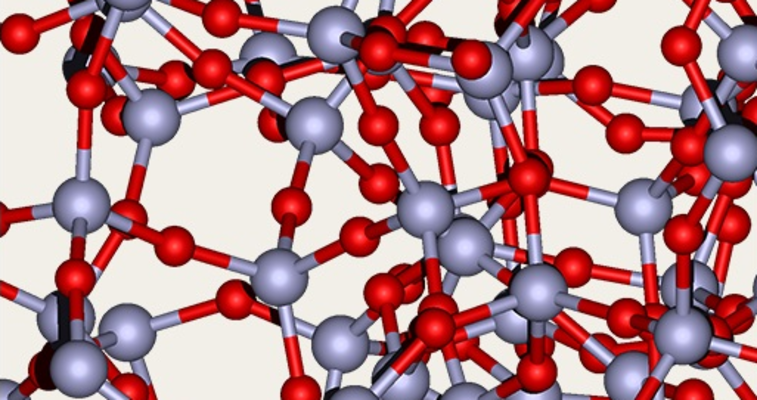
The atoms are connected in a loose, irregular structure
Synthetic amorphous silica is a highly innovative substance that improves countless everyday consumer goods and high-tech products. As an additive, it gives products certain properties. As a processing aid, it ensures that manufacturing processes run smoothly and resource-efficiently.
For example, certain types of silica act as free-flow and anti-caking agents and prevent powders from clumping together. Other types of silica act as carriers for other ingredients, and some can absorb liquids to such an extent that they can be further processed in powder form.
- The food industry needs synthetic amorphous silica to ensure the consistent quality of powdered food products.
- As cleaning particles in toothpastes, silica ensures that teeth are cleaned thoroughly but gently. Silica also serves as a carrier for fluoride.
- Highly porous grades are used, for example, to absorb liquid vitamins and other nutrients and thus allow homogeneous distribution of valuable additives in animal feeds.
- Silica is essential for the automotive industry because it contributes to safe, fuel-saving and long-lasting tires.
Silica is everywhere: Almost every industry – from consumer goods such as toothpaste to high-tech components including microchips – uses synthetic amorphous silica (SAS) as a process aid or functional additive. It is a versatile substance with a wealth of properties. In 95 percent of all applications, silica plays a key role in the function and/or properties of the end product.
In many applications, there are no equivalent alternatives, or developing substitutes and adapting formulations would involve considerable effort, resource consumption and costs.
In many cases, SAS even fulfill several functions at once thanks to their versatile profile of properties: For example, adhesives achieve a firm bond, but do not drip or dry during processing. And in car tires, they increase driving safety, extend durability and reduce fuel consumption. In numerous applications, silicas contribute to sustainability by reducing the waste of resources or extending the service life of products.
Many everyday products, as well as future technologies, would function worse or not at all without silica. In technical terms, the replacement of a proven chemical with a non-equivalent substitute is referred to as a "regrettable substitution."
As a consequence, end consumers will receive products of a poorer quality, while important EU export goods will no longer meet their previous high quality standards and will be harder to sell globally. Some products could even disappear from the market.
Sand is the starting point to produce synthetic amorphous silica (SAS). The manufacturing process converts the inhomogeneous natural raw material into a product with a constantly high quality and purity. This type of silica offers numerous properties for a wide spectrum of specific applications in various industries.
Further information on silica solutions and various applications is available on our website: https://www.silica-specialist.com/en
By two different processes Evonik produces a variety of silica products with customized properties for special applications.
Production of fumed silica (thermal route): A precursor for the silica is dosed into a hydrogen/oxygen flame where fumed silica is formed at temperatures exceeding 1000°C. Evonik's brand name for fumed silica is AEROSIL®.
Production of precipitated silica (wet route): Synthetic amorphous silica is precipitated from an aqueous solution of waterglass (sodium silicate). Evonik's brand names for precipitated silica are SIPERNAT®, SPHERILEX®, ULTRASIL®, ZEODENT® and ZEOFREE®.
Evonik produces a large number of silica products, each of which is optimized for the particular area of application.
Questions regarding functions of silica in various applications
Synthetic amorphous silica (SAS) is used in toothpaste to provide two important functions:
1. As an abrasive to clean and whiten teeth.
2. As a thickener to provide the appropriate rheological properties to the toothpaste.
About 80 % of the toothpaste products marketed in the EU contain silica abrasives. These work during brushing to remove food particles, stains and bacteria from the tooth surface. The properties of thickening silica allow for a toothpaste formulation that can be filled into tubes and remain stable during long term storage. In addition, the toothpaste product when dispersed from a tube, will stand firmly on the toothbrush bristles.
Toothpaste products containing silica can accommodate the most efficacious active ingredients to prevent caries which lead to tooth pain and subsequent tooth loss.
More information on our oral care webpage: https://www.silica-specialist.com/en/our-markets/oral-care
Toothpaste marketers appreciate that ZEODENT® and SPHERILEX® dental abrasives can be manufactured to different levels of cleaning performance. Gentle-cleaning all the way to high-whitening formulas can be built by selecting the appropriate silica abrasive. Optically clear or translucent toothpaste formulations can be achieved by using silica abrasives.
Silica is compatible in toothpaste formulas with anti-cavity fluoride ingredients. These ionic forms of fluoride include sodium fluoride (NaF), stannous fluoride (SnF2) and amine fluorides (AmF). Alternate abrasives are not compatible with ionic forms of fluoride. In addition, anti-gingivitis and anti-sensitivity performance can be achieved in silica-containing toothpaste using stannous fluoride as the active ingredient.
Silica thickeners work synergistically with hydrocolloids (gums) by building a cohesive matrix in the toothpaste product. This matrix provides an optimum rheology to allow batching, filling and long-term stability of toothpaste products, as well as delivering the texture needed for a pleasant consumer experience.
Synthetic amorphous silica (SAS) is approved in the European Union (EU) as a safe food additive and has the identification number E 551.
SAS prevents lumping and caking, and it improves flowability of powdered food such as spices and seasonings, milk powder, egg powder, or instant beverages. Silica is also used for filtration of beer and wine to remove impurities.
By reducing the amount of food loss by caking, silica contributes to the sustainability of the entire food chain. Less food loss also means that the resources used in the run-up to production – for example animal feed, fertilizer, water, effort, and CO2 consumption – were not wasted.
The effects of silica provide numerous advantages within the entire food value chain:
- Food manufacturers benefit from constant product quality and constant dosage of nutrients and flavors. And there is less waste of resources and cleaning downtime caused by caking of powders in production lines.
- Retailers profit from transport and storage stability.
- Consumers enjoy consistent quality and taste as well as convenient handling of free-flowing spices.
More information on our food webpage: https://www.silica-specialist.com/en/our-markets/food
Synthetic amorphous silica acts as flow aids by coating the surface of powder particles, thereby reducing interparticle interactions interspersing and preventing interparticle interactions. By absorbing moisture, fats and oils, silica prevents bridging between particles and reduces stickiness.
Silicon dioxide has been used as a food additive for decades and numerous studies have proven that it is safe to use it in this way. In the European Union (EU), synthetic amorphous silica (SAS) is approved as food additive with the identification number E 551. As with all “E numbers”, this approval is an official guarantee to all consumers that the additive is safe and can be used without any concerns. E 551 is also tested regularly by the European Food Safety Authority (EFSA).
E 551 is one of the most intensively investigated substances and to date there have been no indications of adverse health effects in scientific tests, even with high doses that could not normally be absorbed via food.
The most recent confirmation was given in EFSA’s current scientific opinion on E 551 in 2018. In this statement, it was again confirmed that the use of E 551 in food is safe. Up-to-date intake assessments analyzed the typical amount of silicon dioxide consumers usually ingest with their food. EFSA stated, that the highest exposure estimates of E 551 is much lower than the highest toxicologically tested dose, which still showed no adverse effect.
None of the studies that were conducted according to the valid guidelines of the Organization for Economic Co-operation and Development (OECD), showed any indication of health-damaging effects of SAS on the liver or any other organ system, including the nerve and immune systems.
Evonik’s pharma-grade silica are safe, well-established pharmaceutical excipients that see frequent use. Without silica, the industry would struggle with inaccurate dosage and insufficient bioavailability of active pharma ingredients in drugs as well as with friable tablets.
More information on our health care webpage: https://www.silica-specialist.com/en/our-markets/health-care
The pharmaceutical industry appreciates these key properties of synthetic amorphous silica:
- Free flow of solid ingredients in tableting machines.
- Each tablet contains exactly the same dose of active pharmaceutical ingredients because the improved flowability facilitates precise dosing in tablet presses.
- Tablets come off the tablet press easily during the production process.
- Patients can press their tablets out of the blister without breaking them.
- Orally disintegrating tablets (ODTs) dissolve quickly and without dry mouth feeling.
- Creams, gels, and ointments leave their tubes in perfect consistency.
Over the many decades, synthetic amorphous silica (SAS) has been extensively used as a safe and reliable and safe nature-identical additive in cosmetic and personal care products.
They serve as stabilizers, carrier substances and nature-identical alternatives to microplastics, among other things. Silica gives many cosmetics the desired properties and improves their usability.
SAS is one of the most intensively researched materials. Numerous toxicological studies prove that Evonik’s silica products are suitable and safe for the use in personal care products and cosmetics.
More information on our personal care webpage: https://www.silica-specialist.com/en/our-markets/personal-care
Silica are recognized in the personal care industry for their versatility and effectiveness:
- In creams and lotions, oils and gels, silica helps achieving the desired viscosity which optimizes handling. Silica also creates a pleasant skin feeling, enhances thermal stability and extends shelf-life.
- In hair care and hair styling products, silica absorbs excess sebum, moisturizes hair, or increases volume.
- In decorative cosmetics, silica creates matting and anti-wrinkle (optical blurring) effects.
- In lipsticks, silica improve temperature stability.
- In nail polish, silica prevents dripping as well as settling of pigments.
- Powders become creamy when applied.
- In cleansing lotions and peeling products, silica is a nature-identical alternative to microplastics.
- Silica enables manufacturers to create solid or low-water personal care products.
Synthetic amorphous silica types from Evonik developed specifically for animal feed mainly serve as anti-caking agents for powdery feed and as a carrier substance for nutrients.
Many of the essential ingredients in animal feeds, such as vitamins, organic acids or antioxidants, are liquids. Highly porous silica grades, characterized by their enormous absorption capacity, help to blend liquid ingredients into a powder or granulate animal feed premix so that they can be dosed more precisely. This ensures that livestock as well as pets are accurately and consistently supplied with the necessary nutrients.
With silica as anticaking and free-flow agent, animal feed and its raw materials remain pourable. This is important for easy and resource-efficient manufacturing and processing as well as for storage, transportation, and feed distribution. In this function, silica helps to avoid wasting feed and additives due to caking.
More information on our animal feed webpage: https://www.silica-specialist.com/en/our-markets/feed
Potential economic consequences (Source: EPPA, ‘Socio economic analysis of the Impacts of the Potential Re Classification of Synthetic Amorphous Silica (SAS) as STOT RE 1’
The European industry is a global leader in the production and a net exporter of synthetic amorphous silica (SAS). Around 840,000 tons (2022 estimates) are produced every year in the European Economic Area (EEA). The silica industry is strategically important for the European market and vice versa the European market represents a considerable portion globally. 20 % of the world’s production of SAS is originated from the EEA. As such, the European market is the second largest behind China.
It is estimated that almost half of the EEA production (45 %) is exported outside the EEA.
The demand is still growing at a consistent rate because of the wide range of functions and the specific structural appearances of synthetic amorphous silica. Its use is increasingly important for a number of strategically or economically important and emerging industries.
The downstream user sectors in Europe, using SAS as a high-performance additive, have a combined estimated added value of more than 300 billion EUR per annum.
Source: EPPA, ‘Socio economic analysis of the Impacts of the Potential Re Classification of Synthetic Amorphous Silica (SAS) as STOT RE 1’, Report for Cefic, April 2023
Producers of SAS anticipate a significant loss in demand of SAS (and in turn in sales) as a result of the classification. Most of the economic impacts are expected on the sale of products in highly regulated sectors: food, feed, cosmetics, pharmaceuticals, technical aids.
Moreover, a classification of SAS would compromise sustainability and innovation goals in strategically vital sectors, including automotive, energy production and consumption.
Classification of SAS as STOT RE 1 would therefore have negative impacts on the European economy, innovation, and society overall.
The expected total monetized impact of a classification of SAS as STOT RE 1 represents more than 840 million EUR in the upcoming years until 2028, including economic impacts (EBIT loss) on active substance suppliers, social impacts (i.e., unemployment in the EU-27), economic impacts on downstream users, public health impacts and economic impacts from direct expenditures for consumers.
Source: EPPA, ‘Socio economic analysis of the Impacts of the Potential Re Classification of Synthetic Amorphous Silica (SAS) as STOT RE 1’, Report for Cefic, April 2023
A STOT RE 1 classification of SAS would advantage non-EEA competitors, as well as other substances with similar functions. It would also lead to greater EU dependence on external actors in high-tech industries and to an increase of imports from Asia and the Americas, and greater EU dependence on external actors in high-tech industries.
The EU is a worldwide technological leader in the application of SAS in energy-efficient “green” tires, batteries, renewable energies. One of main drivers for innovation in the silica business is the rapid technological advancement motivated by strong and growing demand for lightweight, functional, robust highly functional materials in electronics, automotive, energy, food packaging, construction, and other industries. A STOT RE 1 classification of SAS would compromise sustainability and innovation goals in a wide range of strategically vital sectors, including automotive, energy production and consumption.
Source: EPPA, ‘Socio economic analysis of the Impacts of the Potential Re Classification of Synthetic Amorphous Silica (SAS) as STOT RE 1’, Report for Cefic, April 2023
A CLP classification of synthetic amorphous silica (SAS) as STOT RE 1 may trigger generic restrictions in Europe: Within the context of the EU Chemical Strategy for Sustainability (CSS), the European Union plans to ban classified substances as of 2028.
Multiple products which utilize SAS could be subject to generic restrictions and bans in the short to medium-long term. Classified substances are either limited or restricted for use in most eco-certified products, for example.
Moreover, products containing substances classified as STOT RE 1 could not be exported outside the EU. This would dramatically reduce the exports of SAS based products to non-EEA markets.
Depending on the severity of the legislative impact of the generic risk approach (GRA), downstream users in key industries may decide to substitute synthetic amorphous silica. But any reformulating process requires costly investments and takes up to ten years’ time. This may result in a price increase for customers.
In many sectors, there is no direct equivalent alternative to SAS on the same quality and performance level. This would invariably be a case of regrettable substitution since the significant advantage of synthetic amorphous silica is that it covers several effects at the same time.
Source: EPPA, ‘Socio economic analysis of the Impacts of the Potential Re Classification of Synthetic Amorphous Silica (SAS) as STOT RE 1’, Report for Cefic, April 2023
Questions regarding silica and safety
The synthetic amorphous silica (SAS) types produced by Evonik are safe for everyone involved in production, processing, transportation, and storage, as well as for consumers of the end products.
At no point in the value chain do people come into contact with silica dust in hazardous concentrations.
Evonik (including its predecessor companies) has been producing SAS since the 1940s. It is one of the most rigorously tested substances regarding potential risks to humans or the environment. Toxicological and ecotoxicological tests and decades of experience in its manufacture and use have resulted in no indications of risks to health or the environment through SAS when the substance is handled appropriately.
Synthetic amorphous silica (SAS) is used in many products and processes. There are no indications of any effects that could damage organs, tissues or genetic material if SAS is breathed in once or repeatedly, even in high doses. No damaging effects on reproduction or development or damage to the immune or nerve system have been reported. As a result, no maximum amounts have been defined for the acceptable daily intake (ADI).
In inhalation studies, SAS caused no long-term changes in the lung or progressive damage comparable to silicosis. In epidemiological studies on employees with longterm exposure, there were also no signs of silicosis; no damage is to be expected under realistic exposure conditions. The available data also contains no indications of lung cancer or other permanent respiratory diseases.
Synthetic amorphous silica can be handled safely when good occupational hygiene and the national or regional applicable maximum allowable concentration are complied with. If this limit value cannot be guaranteed, local extraction equipment must be operated, or dust masks must be worn.
Applicable guiding values at the workplace can be found in the respective safety data sheets of our silica products.
In Germany, for example, a maximum allowable concentration (MAC) of 4 mg/m3 (inhalable) dust must not be exceeded.
This classification is based on tests with rats that led to isolated cases of inflammatory processes in the lung tissue. However, the animals were exposed to disproportionately high amounts of dusts from synthetic amorphous silica (SAS) over a longer period. But the concentration and duration of exposure do not correspond to the real conditions in the production and processing of SAS.
It has also been medically proven that dusts in high concentrations and certain particle sizes generally pose a health risk and are harmful to the respiratory tract. For this reason, general upper limits apply to dusts in workplaces, even if they have no specific toxic effect.
Evonik believes that this classification of synthetic amorphous silica (SAS) as STOT RE 1 is not justified, given that it is the dust particles that may create an adverse effect in the respiratory system and not the chemical (synthetic amorphous silica) itself. Moreover, Evonik is of the opinion that the amount and exposure assumed in the study do not correspond to the real conditions in the handling, industrial processing and any consumer use of SAS.
The proposed classification of synthetic amorphous silica as "harmful to the respratory tract through prolonged or repeated exposure by inhalation" only refers to one extraordinary case: the inhalation of SAS dusts in high concentrations over long periods of time. Consumers do not come into contact with such dusts at all because all consumer products contain silica in bound form only. Safety at workspaces and health of employees is guaranteed by national occupational health and safety regulations.
All necessary instructions for the safe handling and processing of Evonik's silica products can be found in the respective safety data sheets. Even after a STOT RE 1 classification, the occupational safety regulations do not have to be amended.
In Germany, the Technical Rules for Hazardous Substances (TRGS) correspond to the latest standards in occupational medicine and hygiene as well as other established scientific findings for handling hazardous substances, including their classification and labeling.
For amorphous silicas, TRGS 900 sets binding occupational exposure limits for inhalable dust and not the labeling obligation that follows from chemicals legislation.
SAS is only classified as potentially harmful to the respiratory tract in the form of particulate matter. However, there would only be a health risk if dusts were inhaled in large quantities and over a longer period of time without protection.
Safety in production is guaranteed by occupational health and safety regulations. At no stage in production or processing do people come into contact with silica dust in hazardous concentrations.
The use of synthetic additives in food, cosmetics and medicines is strictly regulated in the European Union. Each of them must undergo a rigorous approval process. All types of synthetic amorphous silica (SAS) have been intensively tested for risks and side effects in these applications. SAS have only been approved for use in food, pharmaceuticals, and cosmetics within narrow concentration limits once they have been found to be safe. These approvals are noted in so-called positive lists.
In the regulatory procedures, doses of SAS are evaluated as well as the applications themselves, which can lead to substance exposure. These are therefore complete risk analysis – and not considerations of potential hazards, as in the chemicals legislation. In this respect, application law differs fundamentally from the chemicals legislation, on which the classification of a substance is based.
Food, cosmetics, and pharmaceuticals containing synthetic amorphous silica are therefore safe for consumers.
There may be certain applications of food, cosmetics and pharmaceuticals that generally exclude the use of any type of classified substances. For example, in the case of voluntary self-restrictions by manufacturers or as a requirement of certain eco-labels. However, a classification of synthetic amorphous silica as potentially harmful to the lungs when inhaled does not mean that its use is generally forbidden, because silica only occurs in bound form in end products and this risk does not exist. The approved safety of silica in the applications applies regardless of the STOT RE 1 classification.
The same applies to the processing of food, cosmetics, and pharmaceuticals with silica: the applicable occupational health and safety regulations consider whether and to what extent people are exposed to hazardous substances (exposure). Here too, the focus is on the actual risks and not on potential hazards.
The safety of employees handling synthetic additives is always guaranteed if the national occupational health and safety regulations or instructions in the product safety data sheets are observed.
Exercising environmental responsibility and protecting the health of employees, industrial customers and consumers are integral components of Evonik's business culture. We only produce and market materials if we can manufacture and use them in a safe, environmentally sound manner using our current technology. As part of these efforts, Evonik adheres to the international principles of Responsible Care.
When manufacturing materials, we provide the best possible protection for people and the environment by using closed production systems and additional technical tools, such as filters, vacuum equipment and, when necessary, personal protective equipment. To ensure that these efforts are effective in the workplace, we perform particle counts at regular intervals and provide routine medical care by site physicians.
What is the difference between hazard, risk, and exposure toxicological point of view?
The classification of a chemical can cause uncertainty among consumers because it is not clear to them whether the substance could harm them. For a better understanding, it helps to know the definitions and differences between hazard and risk:
Hazard: A potential source of harm.
A hazard evaluation in toxicology defines what types of harmful effects could occur and under what circumstances (e.g. ingestion, inhalation, skin exposure), and it considers which amount of exposure (dose) is necessary to do so.
Risk: The likelihood that harm from a specific hazard will occur.
Risk Assessment is the formal process of quantifying risk based on known hazards and the amount of exposure. Toxicologists use a mathematical analogy:
Risk = Hazard x Exposure
A good example of this is a pain reliever: A warning on the package says that it can cause damage to the liver. This is a hazard. But that does not mean that any exposure will cause damage. If patients follow the dosing instructions, they can use this pain reliever safely. The risk of damaging the liver increases if they take too much. This is why toxicologists say, “the dose makes the poison”.
Questions regarding nanomaterials
The term comes from the Greek word for dwarf: nanos or nannos. Like the prefixes centi-, milli- or deci-, nano- simply describes an order of magnitude: A nanometer (nm) is a billionth of a meter, i.e., 0.000000001 meters. By way of comparison: A human hair is 50,000 nm thick, an atom is just 0.1 to 0.3 nm and the human genetic substance DNA (desoxyribonucleic acid) has a diameter of approximately 2 nm.
Things as small as this cannot be seen with the human eye and even conventional optical microscopes are not sufficient. Instead, you need special microscopes, such as scanning electron microscopes or transmission electron microscopes (SEM and TEM).
Several different approaches and suggestions could be used to answer this question. A very basic definition is that nanotechnology is concerned with structures and objects of a size between 1 nm and 100 nm in at least one dimension. However, many institutions, researchers and authorities specify different sizes, and no single uniform and universally applicable definition currently exists.
Nanostructured materials are those with a nanoscale structure within the material or at its surface.
Nanomaterials are not dangerous in themselves but are under particular scrutiny in the European Union's substance evaluation.
The scientific risk assessment of the German Federal Institute for Risk Assessment (BfR) focuses on purposefully manufactured nanomaterials. The basic principles of health risk assessment also apply to nanomaterials: Both potential health hazards (harmful effects) and actual exposure must be considered. Due to the wide range of applications of nanomaterials in different products, the uptake pathways via the respiratory tract (inhalation), via the digestive tract (oral) and via the skin (dermal) are considered.
In the EU, most silica products must be labeled as nanomaterials by definition in the product safety data sheet. Some EU countries maintain nano registers in which nanomaterials and their applications must be listed. In some applications and workplace guidelines, other regulations apply that do not define silica products as nanomaterials.
Evonik SAS does not contain isolated nanoparticles; it consists of nanostructured agglomerates. These agglomerates need to be in a specific size range for SAS to fulfill its technical functions as an anti-caking agent and as a flow aid. The nanostructured SAS agglomerates, in turn, are made up of SAS aggregates. These aggregates are formed by inseparably bound nanoparticles
In the EU there are currently different nanomaterial definitions for different areas of law. The relevant definition of "engineered nanomaterials" for food can be found in the Regulation on Novel Foods (EU) 2015/2283, which came into force on January 1, 2018.
The silica used in food as additive E 551 and approved in Regulation (EC) 1333/2008 has been produced and used with the same production processes and product specifications for many decades.
E 551 is not produced to show novel nano properties in food. Rather, silica acts as a spacer between the particles of the powdered food and is therefore approved as an anti-caking agent in Regulation (EC) 1333/2008. Unbound silica primary particles would be too small to act as an anti-caking agent. It is aggregates that fulfill this function. Aggregates normally have dimensions in the micrometer range. No individual primary particles of E 551 have been found in commercially available silica products. Therefore, according to the Food Information to Consumers Regulation (EU) 1169/2011, E 551 does not have to be labeled with the affix “(nano).”
Synthetic amorphous silica (SAS) can enter the body through breathing or with food. However, the body excretes natural amorphous silica and also SAS completely and in an unchanged form.
Dust particles containing silica are caught in the nose when you breathe and are discharged with nasal secretions. Two mechanisms prevent smaller particles from entering the bloodstream: Firstly, the particles are transported out by mucus and cilia, and secondly, the phagocytes continuously clean particles from the lung tissue. Only a very small, negligible part actually gets into the bloodstream, but this is then discharged via the kidneys.
SAS with the classification E 551 is added to food. When this food is eaten, a small part of the silica can be dissolved and get into the bloodstream – this is discharged via the kidneys. The body excretes the remainder normally. No accumulation of SAS in the body has been discovered with any of the ingestion paths.
Most SAS consumed with food is excreted in the stool. Only a very small part is reabsorbed into the bloodstream via the gut in the form of soluble silica and is then discharged again quickly with urine. If silica (E 551) is added to food, in an aqueous environment and depending on the pH, soluble silica can form which can be reabsorbed by the body.
In the mouth cavity and stomach this is not a relevant mechanism for the absorption of silica, even if E 551 is "freely" available after its carrier substance (e.g., salt) has dissolved. The mucous layer in the mouth cavity, which is much thicker (70–100 µm) than the silica particles, creates an effective barrier and protects the oral epithelium; consequently, appreciable resorption via the mouth cavity is practically impossible. Even with a very acidic pH, as found in the stomach, E 551 is not decomposed; soluble silica can only be released and reabsorbed in the small intestine. This small amount is integrated into the body silicon pool.
It is likely that silicon plays a structural role in the formation of connective tissue, including bones and skin. The highest silicon concentrations are found in bones and connective tissue and are higher in a growing organism than in old age. The silicon level in blood is kept constant by resorption and excretion. No accumulation of SAS in the body has been discovered, regardless of how it enters the body.
The cosmetic industry is an important area of application for Evonik's silica products. They get onto the skin with creams and powders, for example. The skin, however, is a very effective barrier to solid particles such as synthetic amorphous silica (SAS). SAS cannot penetrate the top layer of skin.
Skin is a natural barrier that prevents absorption of solid particles such as SAS. Particles applied to the skin have to penetrate several horny layers or, if they bypass this barrier, hair follicles or gland outlets before they could reach living cells in the dermis and get into the systemic circulation. All the information and studies that are available support the view that SAS remains on the skin surface or in the hair follicles and gland outlets and does not penetrate the top layers of skin.
